In a darkened Amsterdam conference hall this summer, a panel of industry and academic scientists took the stage to announce a plan to radically expand the definition of Alzheimer’s disease to include millions of people with no memory complaints.
Those with normal cognition who test positive for elevated levels of certain proteins that have been tied to Alzheimer’s — but not proven to cause the disease — would be diagnosed as having Alzheimer’s Stage 1, the panel members explained.
Even before the presentation ended, attendees in the packed hall were lining up behind microphones to ask questions, according to video of the event.
“I’m troubled by this,” Dr. Andrea Bozoki, a University of North Carolina neurologist, told the panel. “You are taking a bunch of people who may never develop dementia or even cognitive impairment and you’re calling them Stage 1. That doesn’t seem to fit.”
Under the proposal, tens of millions of Americans with normal cognition would test positive for abnormal levels of amyloid or tau, the two proteins the tests look for, and the majority of them may never be diagnosed with dementia, studies suggest. A 60-year-old man who tests positive, for example, is estimated to have a 23% risk of developing dementia in his lifetime.
Criticism of the plan has intensified since it was unveiled in July at the international conference attended by 11,000 doctors and scientists. But the panel, organized by the nonprofit Alzheimer’s Assn., is continuing its push to extend the diagnosis to people who have no problem recalling events or what day it is — and convince skeptics that Alzheimer’s symptoms aren’t necessary to have the disease.
Panel members argue that the earlier patients get help, the more effective it might be. The availability of new drugs for patients with early Alzheimer’s symptoms has spurred them into action now, they say.
The plan could be approved by the panel and published in a medical journal early this year, association officials said. Such a move is likely to be influential: A similar proposal in 2018 that was put forth to help guide research on experimental Alzheimer’s medications was quickly adopted by the Food and Drug Administration and is frequently cited by doctors, scientists and health insurers.
Standing to benefit are the pharmaceutical and medical testing companies who employ seven members of the 20-person panel. At least seven more members of the panel are academics who receive money from those companies for consulting or research. Panelists reached by The Times said the funding did not influence their decisions.
Four other scientists who are outside advisors to the panel are executives from Eisai and Biogen, the makers of two new medicines for Alzheimer’s patients, and Eli Lilly and Genentech, which are developing similar drugs.
The American Geriatrics Society called the panel members’ financial ties to industry “wholly inappropriate.” In an analysis of the proposal, the society warned the proposal could lead to overdiagnosis of Alzheimer’s and subject people to treatments with “limited benefit and high potential for harm.”
Others said the plan was premature at best.
“I think this is untested, uncharted territory,” said Dr. Madhav Thambisetty, a senior researcher at the National Institute of Aging. “I’m not at that stage where I would be able to make a diagnosis of Alzheimer’s disease in somebody who’s cognitively normal based on the presence of a single biomarker.”
A researcher at Washington University in St. Louis works on a blood test for Alzheimer’s disease.
(Huy Mach / University of Washington via Associated Press)
Under the proposal, people with no memory problems who test positive for abnormal levels of amyloid or tau proteins would be classified as Stage 1. They would move to Stage 2 if they begin to experience “neurobehavioral difficulties” such as depression, anxiety or apathy — symptoms often unrelated to Alzheimer’s — even if the patient’s cognition is unchanged.
Stage 3 would be for those with mild cognitive impairment, while Stages 4 through 6 would describe patients with mild, moderate or severe dementia.
The move to label more Americans as having Alzheimer’s comes amid a decades-long decline in the risk of dementia. Researchers don’t know why the risk is falling, but they say higher levels of education, a reduction in smoking and better treatment of high blood pressure could all be factors.
Dr. Peter Whitehouse, professor of neurology at Case Western Reserve University, is one of several doctors who have noted that the plan could benefit the Alzheimer’s Assn. since the majority of its donations come from people who know one of the estimated 6.7 million Americans now living with the disease and want to help find a cure. If more Americans are diagnosed with the disease under the new definition, the ranks of possible donors would swell, he said.
“This raises the potential for more people to want to give money,” Whitehouse added.
The panel said it was proposing the changes now because the FDA has approved two drugs — Eisai’s Leqembi and Aduhelm from Biogen — for patients in the early stages of memory decline. While a study of Leqembi’s effects on asymptomatic people has begun, there is currently no evidence that giving it to people without cognitive impairment can reduce the risk of dementia or delay the onset of Alzheimer’s symptoms.
Another reason for the change, the panel said, was the availability of new blood tests that do an “excellent” job of detecting abnormal levels of amyloid and tau in the brain. The blood tests are easier and less invasive than the PET scans and spinal taps that traditionally have been used to measure levels of Alzheimer’s-related proteins.
“The purpose of this initiative is to advance the science of early detection and treatment,” said panel member Maria Carrillo, the Alzheimer Assn.’s chief science officer. “In order to prevent dementia, we need to detect and treat the disease before symptoms appear.”
Thambisetty and other doctors also note that the plan does not address the serious bioethical concerns that come with testing healthy people for signs of Alzheimer’s.
People with no memory problems who learn they are positive for abnormal levels of amyloid or tau proteins can suffer from depression, anxiety and thoughts of suicide, studies have found.
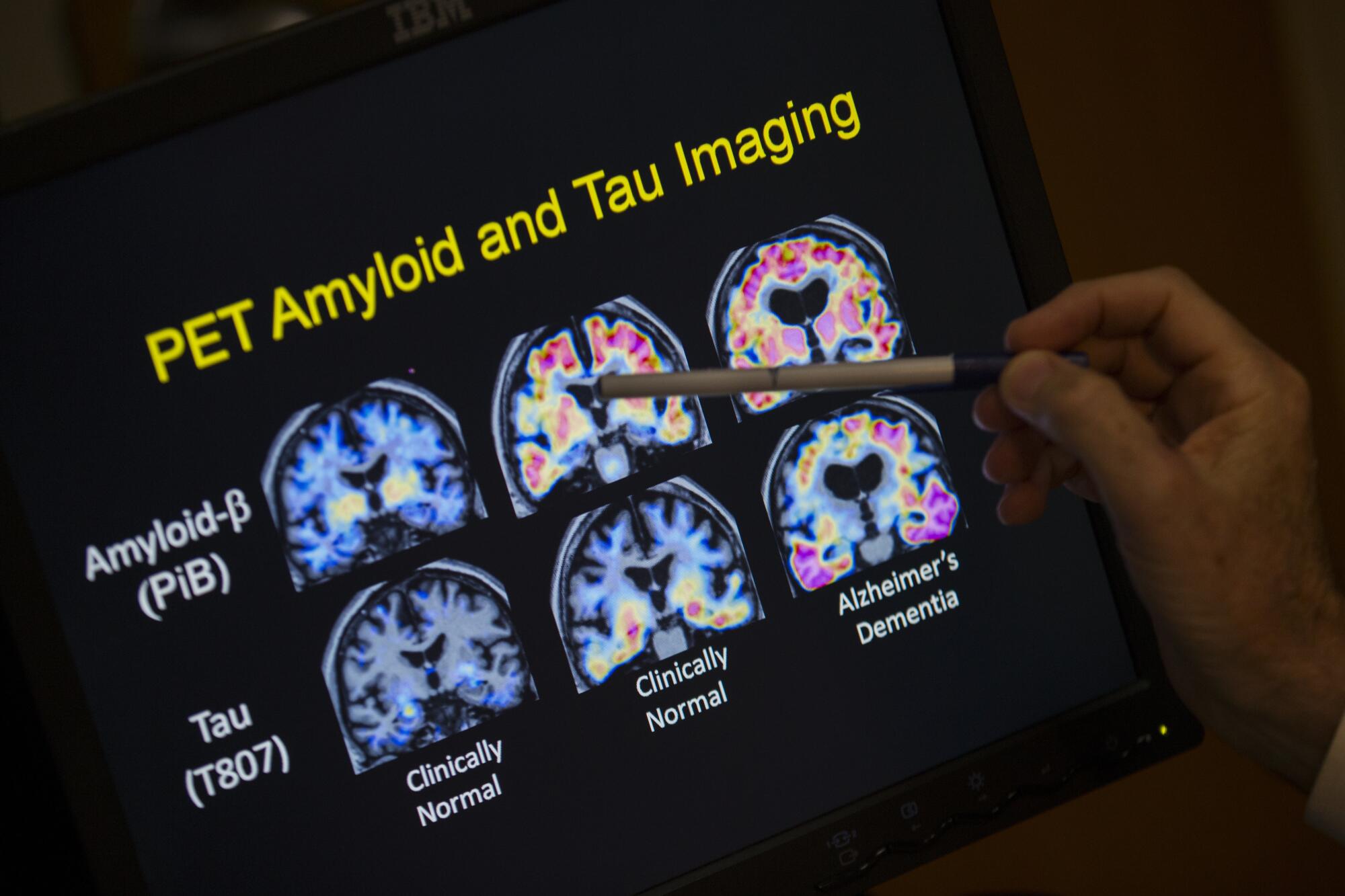
A doctor points to PET scan results that are part of a study on Alzheimer’s disease at a hospital in Washington.
(Evan Vucci / Associated Press)
A positive test can also lead to discrimination by employers and by companies offering life, disability and long-term care insurance. That risk is so real that people with no memory complaints who volunteer for an ongoing clinical trial that requires an amyloid test are advised to consider getting any insurance they’ve been contemplating before taking the test.
“This is an ethically gray area,” Thambisetty said of testing cognitively normal people. “There are many questions that remain to be answered.”
Added Dr. Eric Widera, a geriatrician at UC San Francisco: “If somebody tests positive for amyloid and they are an airplane pilot, do they have to disclose that to the airlines? They are not asking these questions.”
Concerns like these led the panel members to revise the draft to say they were not yet advocating for “routine” testing of those without memory problems. And Dr. Clifford R. Jack Jr., a radiologist at the Mayo Clinic who leads the panel, told The Times the proposal was not an instruction manual to guide doctors in the evaluation, diagnosis and treatment of their patients.
“Should you diagnose Alzheimer’s disease in asymptomatic persons? The answer is no,” Jack said.
The changes did not reassure skeptics.
Widera pointed out that under the revised plan, an unimpaired person who tests positive for an Alzheimer’s biomarker would not be considered “at risk” for the disease because — in the panel’s view — they already have it.
“They are redefining what it means to have Alzheimer’s,” he said. “You no longer need to have cognitive impairment to have this disease. You just need the positive blood test.”
They are redefining what it means to have Alzheimer’s.
— Dr. Eric Widera, a geriatrician at UC San Francisco
That could lead doctors to prescribe the new drugs to people without memory problems, Widera said.
Indeed, interest in testing for Alzheimer’s-related proteins exploded after the FDA controversially approved Aduhelm and Leqembi, which reduce amyloid levels in the brain.
The hypothesis is that finding amyloid early and removing it might avoid irreversible brain damage. But so far researchers have failed to demonstrate that a build-up of amyloid causes dementia — or that removing it alleviates symptoms.
The FDA went against the advice of its independent advisory committee and green-lighted Biogen’s Aduhelm in 2021 even though there was a lack of evidence that it reduced cognitive decline. A Congressional investigation later found that Biogen executives met with FDA officials — including Dr. Billy Dunn, head of the neuroscience office — dozens of times and inappropriately collaborated on a key regulatory document. Dunn did not respond to questions from The Times.
The FDA approved the second drug, Eisai’s Leqembi, in July after a study showed it could slow the progression of Alzheimer’s in people with mild cognitive impairment by less than half a point on an 18-point scale, a finding that some doctors doubt would be noticeable to patients or their families.
The agency requires both drugs to carry warnings that they can cause potentially fatal bleeding or swelling in the brain.
A closeup of a human brain affected by Alzheimer’s disease is on display at the Museum of Neuroanatomy at the University at Buffalo in Buffalo, N.Y.
(David Duprey / Associated Press)
The Alzheimer’s Assn. has been among the most vocal advocates for the two drugs, which each cost more than $26,000 a year. The group deployed hundreds of volunteers to lobby Congress and get Medicare to pay for the treatments.
While prescriptions of Leqembi are now taking off, doctors have hesitated to prescribe Aduhelm. Last month, Biogen said it planned to stop selling Aduhelm and instead focus on promoting Leqembi through its partnership with Eisai.
The Alzheimer’s Assn.’s plan to create a new class of symptom-free Alzheimer’s patients began taking shape more than a decade ago and was included in proposals to update diagnostic criteria for the disease in 2011 and 2018.
The association’s website says the idea came from a meeting of its Research Roundtable, a group that companies pay thousands of dollars to join. The roundtable meets twice a year, often at the luxury Park Hyatt Hotel in Washington, D.C. Current members include Biogen, Eisai, Lilly, Genentech, Prothena and 15 other companies. Selected academics and drug regulators from around the world are also invited to attend.
In its 2023 fiscal year, the Alzheimer’s Assn. received $4.9 million from pharmaceutical, biotech, diagnostic and clinical research companies — more than in any of the previous five years. The association said those corporate donations amount to just 1.3% of its total cash donations of $379 million that year.
Carrillo, the association’s chief science officer, told The Times in a statement that “no contribution from any organization impacts the Alzheimer’s Association decision-making, nor our positions.”
“We make our decision based on science, and the needs of our constituents,” she said.
The association spent $100 million on research in its 2023 fiscal year, including grants to some of the academic scientists on the panel or to the universities they work for. Many of those grants are aimed at creating new strategies for early diagnosis of people without memory complaints.
That message of early detection is echoed by pharmaceutical and testing companies. At a scientific conference in Boston in October, Dr. Mark Mintun, an Eli Lilly executive who is an advisor to the panel, said in a presentation that the company’s experimental medicine donanemab helped younger people and those with lower levels of tau more than it helped older people and those with higher levels of the protein.
“This gives us great urgency in thinking about how to diagnose and prepare patients for treatment,” Mintun told the audience, according to a report on the Alzforum news website.
Among the seven industry executives sitting on the Alzheimer’s Assn. panel are former FDA official Dunn, who is now on the board of Prothena, a company developing anti-amyloid drugs; Dr. Eric Siemers, chief medical officer of Acumen Pharmaceuticals, which is also working on anti-amyloid drugs; and Dr. Philip Scheltens, who heads a venture capital fund that invests in dementia drugs.
They are joined by Dr. Reisa Sperling, a Harvard neurology professor who has received research grants from Eisai and Lilly and consulting fees from 18 other companies, according to the panel’s disclosures.
Sperling has led studies investigating the value of treating people without memory problems. She said in 2013 that she could see a future where “we will treat everybody preemptively, in the same way we vaccinate.”
Other academic panel members include Charlotte Teunissen, a professor at Amsterdam University Medical Centers who conducts research for 25 companies, and Dr. Michael Rafii, a USC professor of clinical neurology, who disclosed work for 11 companies.
Both Teunissen and Rafii said their industry funding has no bearing on their judgment.
“I believe working with a diverse group of pharmaceutical and biotech companies, each with their own therapeutic approaches and strategies, can mitigate against a single company’s influence,” Rafii said.
Sperling agreed that corporate research funding did not affect her objectivity. “I want to figure out the truth,” she said.
But others are not convinced.
“This panel is dominated by those with financial ties to companies that will directly benefit” from a more expansive view of Alzheimer’s, said Widera of UCSF. “And there was no consideration about the potential downsides or risk to the number of people who are going to be now diagnosed” if its definition is adopted.
The proposal — initially dubbed “The National Institute of Aging–Alzheimer’s Association Revised Criteria for Diagnosing and Staging Alzheimer’s Disease” — has received international attention in part because it seemed to have the backing of one of the U.S. government’s premiere research centers.
The American Geriatrics Society and others said the proposal’s name implied that the NIA, which is part of the National Institutes of Health, was a full partner in the effort.
But Dr. Eliezer Masliah, director of the institute’s neuroscience division, said that while he and another NIA scientist attend panel meetings, they are not involved in its decisions. “We’re listening and recording and just keeping track of the process,” he said.
After The Times asked NIH officials about the NIA’s involvement, they said the institute’s name would be removed from the proposal’s title.
Even before the plan has been finalized, one company told investors it was poised to benefit.
In a November call with Wall Street analysts, Masoud Toloue, the chief executive at Quanterix, pointed out that the company’s blood test for tau — called p-Tau 217 — had been recommended by the panel for diagnosing the disease.
“We believe we’re in a strong position to capitalize on these opportunities,” Toloue said.


